Adsense
Raji Chem World Search Engine

Custom Search
Find the Properties of Chemicals
Nature Chemistry
Nature Chemistry
Adsense
Nature Chemistry
Thursday, December 29, 2011
Tuesday, December 27, 2011
Friday, December 23, 2011
Tuesday, December 20, 2011
Thursday, December 15, 2011
Silanol as a Removable Directing Group for the PdII-Catalyzed Direct Olefination of Arenes

DOI: 10.1002/chem.201103171
Synthesis of chromans via Pd-catalyzed alkene carboetherification reactions

A new method for the construction of 2-substituted and 2,2-disubstituted chromans via Pd-catalyzed carboetherification reactions between aryl/alkenyl halides and 2-(but-3-en-1-yl)phenols is described. These reactions effect formation of a C–O bond and a C–C bond to afford the chroman products in good yield, and also provide stereoselective access to tricyclic chroman derivatives.
Chem. Commun., 2012, 48, 609-611
Pd-Catalyzed Multiple C[BOND]H Functionalization to Construct Biologically Active Compounds from Aryl Aldoxime Ethers with Arenes

- Chien-Hong Cheng, DOI: 10.1002/chem.201102996
Nickel- and Cobalt-Catalyzed Direct Alkylation of Azoles with N-Tosylhydrazones Bearing Unactivated Alkyl Groups

- Masahiro Miura, DOI: 10.1002/anie.201106825
Transition-Metal-Free Direct Arylation of Anilines

Aryne arylation: A new method of direct arylation is reported for aniline substrates. The reaction uses benzyne to synthesize a variety of aminobiaryls under mild conditions (see scheme), requiring no stoichiometric metalation or transition-metal catalysis. An ene mechanism is implicated, and conveys excellent functional group tolerance relative to metal-mediated processes.
DOI: 10.1002/anie.201106150
Palladium-Catalyzed Oxidative C[BOND]H Bond Acylation of Acetanilides with Benzylic Alcohols

An efficient and clean method to construct C![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) C bonds has been developed via the acylation reaction of acetanilides with benzylic alcohols using tert-butyl hydroperoxide (TBHP) as oxidant catalyzed by palladium acetate in the presence of triflic acid (TfOH). The acylation reactions exhibit excellent reactivities, and up to 95% yield of the corresponding aryl ketone could be obtained under the optimal conditions.
C bonds has been developed via the acylation reaction of acetanilides with benzylic alcohols using tert-butyl hydroperoxide (TBHP) as oxidant catalyzed by palladium acetate in the presence of triflic acid (TfOH). The acylation reactions exhibit excellent reactivities, and up to 95% yield of the corresponding aryl ketone could be obtained under the optimal conditions.
![[BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8ff.gif) C bonds has been developed via the acylation reaction of acetanilides with benzylic alcohols using tert-butyl hydroperoxide (TBHP) as oxidant catalyzed by palladium acetate in the presence of triflic acid (TfOH). The acylation reactions exhibit excellent reactivities, and up to 95% yield of the corresponding aryl ketone could be obtained under the optimal conditions.
C bonds has been developed via the acylation reaction of acetanilides with benzylic alcohols using tert-butyl hydroperoxide (TBHP) as oxidant catalyzed by palladium acetate in the presence of triflic acid (TfOH). The acylation reactions exhibit excellent reactivities, and up to 95% yield of the corresponding aryl ketone could be obtained under the optimal conditions.
DOI: 10.1002/adsc.201100417
User-Friendly [(Diglyme)NiBr2]-Catalyzed Direct Alkylations of Heteroarenes with Unactivated Alkyl Halides through C[BOND]H Bond Cleavages

- Lutz Ackermann, DOI: 10.1002/adsc.201100487
Wednesday, December 14, 2011
Palladium-Catalyzed Selective Synthesis of Naphthalenes and Indenones and Their Luminescent Properties - Chen - 2011 - European Journal of Organic Chemistry - Wiley Online Library

Selective synthesis of functionalized naphthalenes and indenones by palladium-catalyzed cyclization of o-haloacetophenones and terminal alkynes in the presence of a secondary amine is reported. Under a nitrogen atmosphere, the palladium-catalyzed reaction of o-haloacetophenones with terminal alkynes in the presence of wet secondary aminesgenerated 1-(dialkylamino)-3-aryl/alkylnaphthalenes. When the reaction was conducted in air, 1-indenone-3-carbaldehydes were obtained. The synthesized β-arylnaphthalenes 4a–f emit light in a range from 386 to 452 nm with quantum yields from 0.10 to 0.48 in cyclohexane. The absorption and emission efficiency of 4d were finely tuned by the acidity of the environment, and this might be useful in the use of 4d as a fluorescent pH sensor.
Copper-Mediated C–H Activation of 1,3,4-Oxadiazoles with 1,1-Dibromo-1-alkenes Using PEG-400 as a Solvent Medium: Distinct Approach for the Alkynylation of 1,3,4-Oxadiazoles

The direct C–H alkynylation of 1,3,4-oxadiazoles with 1,1-dibromo-1-alkenes has been accomplished by using a combination of CuBr/LiOtBu in PEG-400 (a green solvent) at 80 °C. The products were formed in high yields (73–86 %) in 2 h. No additional ligand or volatile solvent was required and the conversion was ecofriendly.
DOI: 10.1002/ejoc.201101542
Direct amide formation from unactivated carboxylic acids and amines
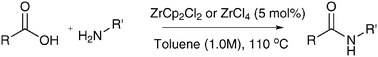
The direct coupling of unactivated carboxylic acids with amines can be performed in toluene 110 °C in the absence of catalyst. The use of simple zirconium catalysts at 5 mol% loading gave amide formation in as little as 4 h.
Chem. Commun., 2012, 48, 666-668
Friday, December 9, 2011
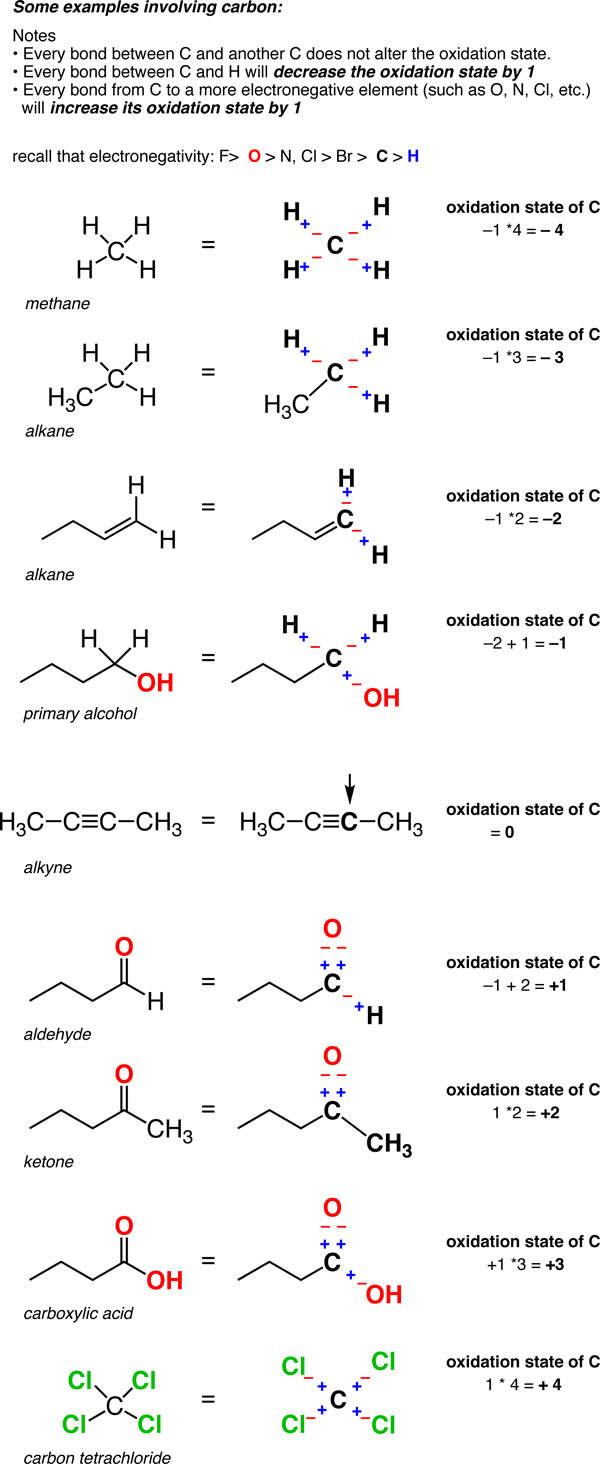
Oxidation State of Carbon
Calculating oxidation states of different metals should be pretty familiar. Here’s what you do. Take a typical compound – FeCl3, for instance. Treat every bond between the metal and a different atom as if it were an ionic bond. That means the more electronegative elements (like chlorine, say, or oxygen) bear negative charges, and the less electronegative element (such as the metal) bears the positive charge.
If the compound is neutral, the sum of the oxidation states also has to be neutral. (If the compound has a charge, you adjust the oxidation states accordingly so that their sum equals the charge).

Now here’s a fun exercise. Try applying the same rules to carbon.
It’s going to feel a little bit weird. Why? Because there are two key differences.
- First, carbon is often more electronegative (2.5) than some of the atoms it’s bound to (such as H, 2.2). So what do you do in this case?
- Secondly, unlike metal-metal bonds, carbon-carbon bonds are ubiquitous. So how do you deal with them?
Two answers.
- In a C-H bond, the H is treated as if it has an oxidation state of +1. This means that every C-H bond will decrease the oxidation state of carbon by 1.
- Any two bonds between the same atom do not affect the oxidation state (recall that the oxidation state of Cl in Cl-Cl (and that of H in H-H) is zero. So a carbon attached to 4 carbons has an oxidation state of zero.
So unlike metals, which are almost always in a positive oxidation state, the oxidation state of carbon can vary widely, from -4 (in CH4) to +4 (such as in CO2). Here are some examples.
(Don’t forget that this is called a “formalism” for a reason. The charge on the carbon isn’t really+4 or –4. But the oxidation state formalism helps us keep track of where the electrons are going, which will come in handy very soon).
 |
| Oxidation States of Carbon |
 |
| Oxidation States of Carbon |
Thursday, December 8, 2011
Wednesday, December 7, 2011
Mild chemo-selective hydration of terminal alkynes catalysed by AgSbF6

Mathieu Bui The Thuong, André Mann and Alain Wagner
Chem. Commun., 2012, 48, 434-436
DOI: 10.1039/C1CC12928G
Selective reductive transformations using samarium diiodide-water
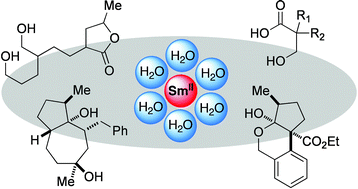
Michal Szostak, Malcolm Spain, Dixit Parmar and David J. Procter
Chem. Commun., 2012, 48, 330-346
DOI: 10.1039/C1CC14252F
Tuesday, December 6, 2011
Subscribe to:
Comments (Atom)