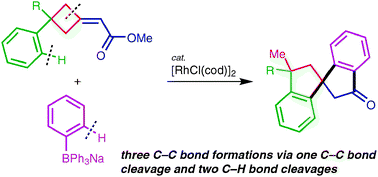
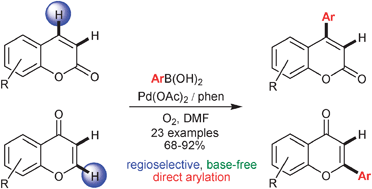
This Minireview provides a critical account of the development of allene-containing advanced functional materials, starting with the design and synthesis of stable and enantiopure building blocks. A variety of systems, including shape-persistent macrocycles, foldamers, polymers, charge-transfer chromophores, dendrimers, liquid crystals, and redox-switchable chiral chromophores are discussed from the viewpoint of their syntheses, properties, and potential applications. The goal of this Minireview is to inspire new uses of enantiopure allenes for the rational design of advanced materials.





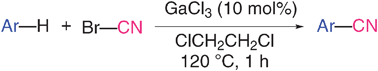
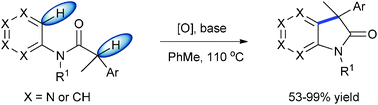









 \
\