Adsense
Raji Chem World Search Engine

Custom Search
Find the Properties of Chemicals
Nature Chemistry
Nature Chemistry
Adsense
Nature Chemistry
Friday, February 3, 2012
Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates
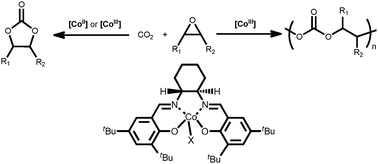
This comprehensive tutorial review focuses on well-defined cobalt complexes that serve as homogeneous catalysts for the production of polycarbonates and cyclic carbonates from the coupling of carbon dioxide and epoxides. Special considerations are given to the mechanistic pathways involved in these processes.
Green chemistry oriented organic synthesis in water
The use of water as solvent features many benefits such as improving reactivities and selectivities, simplifying the workup procedures, enabling the recycling of the catalyst and allowing mild reaction conditions and protecting-group free synthesis in addition to being benign itself. In addition, exploring organic chemistry in water can lead to uncommon reactivities and selectivities complementing the organic chemists' synthetic toolbox in organic solvents. Studying chemistry in water also allows insight to be gained into Nature's way of chemical synthesis. However, using water as solvent is not always green. This tutorial reviewbriefly discusses organic synthesis in water with a Green Chemistry perspective.
Thursday, February 2, 2012
Oxidative Prins and Prins/Friedel–Crafts cyclizations for the stereoselective synthesis of dioxabicycles and hexahydro-1H-benzo[f]isochromenes via the benzylic C–H activation
![Graphical abstract: Oxidative Prins and Prins/Friedel–Crafts cyclizations for the stereoselective synthesis of dioxabicycles and hexahydro-1H-benzo[f]isochromenes via the benzylic C–H activation](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C1OB06489D)
1-Benzyl ethers of (E)- and (Z)-hex-3-en-1,6-diols and hept-3-en-1,7-diols undergo a smooth oxidative cyclization with DDQ in the presence of In(OTf)3 through a sequential C–H bond activation and an intramolecular Prins cyclization to afford the corresponding trans- and cis-fused hexahydro-2H-furo[3,2-c]pyrans and octahydropyrano[4,3-b]pyrans respectively in good yields with an excellent stereoselectivity. Aryl tethered homoallylbenzyl ethers such as benzyl ethers of (E)- and (Z)-6-arylhex-3-enyl alcohols undergo a tandem Prins/Friedel–Crafts cyclization in the presence of stoichiometric amounts of DDQ and SnCl4via the benzylic C–H bond activation to furnish the corresponding trans- and cis-fused hexahydro-1H-benzo[f]isochromenes in good yields with complete stereoselectivity.
Tuesday, January 31, 2012
Iron-Catalyzed Regio- and Stereoselective Chlorosulfonylation of Terminal Alkynes with Aromatic Sulfonyl Chlorides

Terminal alkynes react with aromatic sulfonyl chlorides in the presence of an iron(II) catalyst and a phosphine ligand to give (E)-β-chlorovinylsulfones with 100% regio- and stereoselectivity. Various functional groups, such as chloride, bromide, iodide, nitro, ketone, and aldehyde, are tolerated under the reaction conditions. Addition of tosyl chloride to a 1,6-enyne followed by radical 5-exo-trig cyclization gave an exocyclic alkenylsulfone.
Subscribe to:
Posts (Atom)









