The author report an intermolecular hydroacylation of allylic alcohols with salicylaldehydes to afford β-hydroxy aryl ketones in 73–94% yields as single regioisomers. The reactivity and regioselectivity were promoted by catalytic amounts of a phosphinite that forms a dynamic covalent bond with the allylic alcohol. Hydroacylation of 1,2- and 1,1-disubstituted olefins generates β-hydroxy aryl ketones bearing tertiary and quaternary centers, respectively.
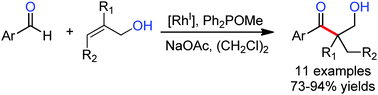

No comments:
Post a Comment